Why manufacture medical devices in the UK?
With numerous breakthroughs in technology and applications, the UK remains one of the most promising places for medical device manufacturing.
The UK medical device market
According to the government, the medical device market in the United Kingdom is the sixth largest worldwide and the third largest in Europe. The medical technology industry in the United Kingdom brings in about $33 billion annually, consisting mostly of small and medium-sized businesses located across the nation, with hubs of activity in the Midlands and Southeast of England. Numerous multinational corporations, such as prominent American producers of medical technology, have headquarters or production plants in the United Kingdom. Furthermore, the Academic Health Science Network (AHSN) reported that within medtech (the creation, production, and distribution of medical equipment), 53 major enterprises with a revenue of over £50 million are driving the UK market ahead, 84% of all businesses active in this area are SMEs.
Approximately 86% of the nation’s healthcare is provided by the National Health Service (NHS), which receives funding from the government and is the main buyer of medical equipment. In England, 83% of the population receives care via the NHS. Acute hospital trusts, which spend an estimated $13 billion annually on medical equipment, handle the majority of its procurement.
Benefits of manufacturing medical devices in the UK
- Quality control: Medical device manufacturers in the UK must comply with stringent regulations, with most certified to ISO 13485 and other high-quality standards. Local manufacturing offers UK customers greater control over operations, quality management, and the final product. Additionally, shorter transportation distances reduce supply chain risks and lower shipping costs.
- Local knowledge and expertise: Local UK medical device manufacturers have the best knowledge and experiences in understanding the UK medical device market, hence can give their clients valuable insights of the market, leading to improved product designs, new distribution opportunities, etc.
- Environmental benefits: For medical devices to be sold in the UK, manufacturing locally saves mileage, fuel cost, delivery times, hence reducing other environmental costs.
- Customer support: UK-based medical device manufacturers can provide rapid and efficient support, particularly for clients looking to distribute products within the UK. By collaborating with key customers like the NHS, these manufacturers offer ongoing, high-quality support that international counterparts may struggle to match.
- Support the local economy: Manufacturing medical devices in the UK has significant potential to boost the economy by creating jobs, providing training opportunities, generating income for suppliers (such as toolings and raw materials), and contributing to overall economic growth.
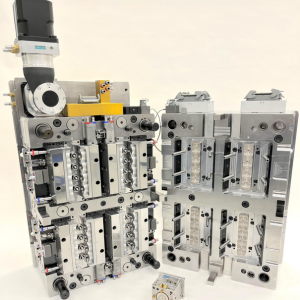
Medical moulds of various cavities and size manufactured at Micro Systems, a UK mould maker and injection moulder.
Great government support
The UK government has spent billions of pounds on boosting the British manufacturing sector. Recently, the UK government just announced £4.5 billion in funding for British manufacturing to increase investment in eight sectors across the UK, including £520 million for life sciences manufacturing that would help the UK leverage on its world-class research and development while strengthening its preparedness for future medical emergencies. This huge investment aims to ensure the UK is the ideal place for manufacturers to start, grow and further invest in their manufacturing activities.
In the recent Medical technology strategy, the UK government also commits to further strengthen the MedTech industry in the UK. The goal of this approach is to promote the creation and uptake of cutting-edge medical technology. Businesses who manufacture cutting-edge, specialty medical products and may encounter difficulties in the wider EU market would especially benefit from this simplified process.
Micro Systems has more than 20 years of experience in mould design, mould manufacture and injection moulding for medical devices in the UK. Micro Systems is ISO 9001 and ISO 13485 certified, with extensive knowledge and experiences about the medical device market in the UK and EU.
Contact us today to discuss your medical device project!





