Unlocking Precision in Medical Manufacturing with Variotherm Injection Moulding
In the highly regulated and quality-driven world of medical device manufacturing, every detail counts. From flawless surface finishes to the faithful replication of microscopic features, the bar is constantly being raised for plastic components used in diagnostics, drug delivery, and surgical applications.
What Is Variotherm Injection Moulding?
Variotherm (short for variable thermal control) is a moulding process that employs rapid, cycle-synchronous heating and cooling of the mould cavity surface. Instead of maintaining a constant temperature, the mould temperature is raised during the injection phase and rapidly cooled prior to part ejection. This thermal modulation is tightly controlled via advanced temperature control units (TCUs), using mediums such as pressurised water, steam, or induction heating.
Injection moulding opens door to endless opportunities in Medical applications (Photo: Micro Systems)
Process Workflow of Variotherm Injection Moulding
- Mould Heating (Pre-Fill): The mould surface is rapidly heated to a high temperature to allow the melt front to flow more uniformly, delaying freeze-off and eliminating weld lines or flow marks.
- Injection Phase: Thermoplastic resin (e.g. PC, PMMA, PEEK) is injected under high pressure into the heated mould. The molten polymer replicates microstructures and critical geometry with improved fidelity.
- Mould Cooling (Post-Fill): Once the cavity is filled, the mould is rapidly cooled to solidify the part and achieve a stable demoulding condition. This is done through automated switching of heating and cooling circuits.
This time-temperature profile is programmed and monitored in real time, with closed-loop control to ensure repeatability and process stability.
Applications of Variotherm Injection Moulding in Medical Devices
Variotherm Injection Moulding are applied for components that require exceptional surface integrity and micro-feature replication, such as:
- Microfluidic cartridges and test strips
- Inhaler and autoinjector components
- Diagnostic housing covers with optical windows
- Snap-fit surgical enclosures
- Laser-marked or decorated parts requiring zero flow marks
By removing visible defects and ensuring reproducibility, this process reduces downstream inspection, rework, and assembly issues—resulting in lower total cost of quality.
Technical Advantages for Medical Applications
Variotherm is particularly well-suited for complex medical device parts where quality cannot be compromised:
1. Superior Surface Finish
- Eliminates sink marks, weld lines, and flow marks.
- Achieves optical-grade transparency for diagnostic windows or housings.
- Supports mirror finishes and high-gloss surfaces directly off-tool.
2. Improved Mould Filling and Flow Balance
- Delays skin formation and freeze-off, allowing the melt to fully fill sharp corners, microchannels, or thin-walled sections without hesitation.
- Enhances flow length by 30–100%, depending on polymer and wall thickness.
3. Higher Replication Fidelity
- Critical for microfluidic features, capillary structures, and lens geometries.
- Allows full replication of sub-50 µm features with minimal distortion.
4. Reduced Residual Stress
- Lower internal stress improves dimensional stability and long-term part performance.
- Particularly beneficial for load-bearing components and ultrasonic weld zones.
Materials Compatibility
Variotherm is compatible with a wide range of medical-grade thermoplastics, including:
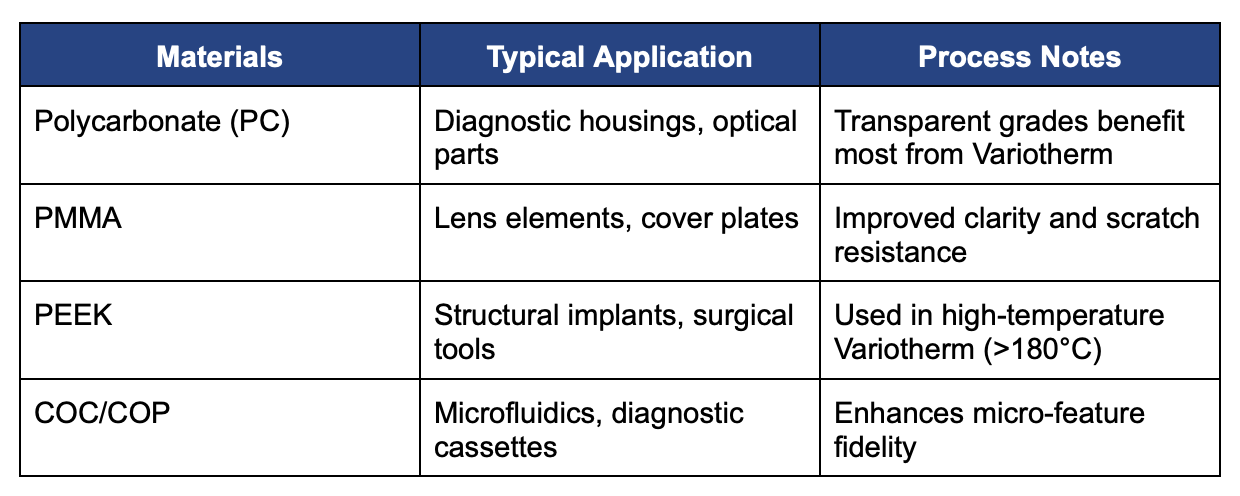
All materials used are USP Class VI and ISO 10993 compliant.
Validation and Quality Assurance
In alignment with our ISO 9001:2015 and ISO 13485:2016 certifications, all Variotherm-enabled moulding processes are fully validated, traceable, and controlled under Quality Management System.
Validation includes:
- IQ/OQ/PQ for each tool and material
- Gage R&R and CpK studies for critical dimensions
- In-line process monitoring (e.g. cavity pressure, melt temperature)
- SPC and electronic batch records (EBR)
Quality metrics achieved:
- Surface roughness Ra < 0.3 μm
- Dimensional tolerances to ±0.01 mm
- Weld line tensile strength >90% of bulk material
Process data is archived for a minimum of 10 years and fully traceable to part and batch level, in compliance with FDA 21 CFR Part 820 and EU MDR requirements.

Micro Systems is ISO 9001 and ISO 13485 certified, with full capabilities to support your most challenging injection moulding projects
If you’re developing a high-performance medical component—or experiencing issues with weld lines, surface finish, or dimensional variation—our engineering team would be happy to evaluate whether Variotherm Injection Moulding is right for your application.
Contact us today!





