Tooling and Moulding for Point-of-Care Diagnostic Devices
Point‑of‑Care (PoC) diagnostic devices—such as lateral‑flow test cartridges, microfluidic cassettes, and biosensor housings—demand ultra‑precise, biocompatible, and sterile plastic components. Tooling and moulding are therefore central to achieving performance, regulatory compliance, scalability, and cost‑efficiency.
1. Material Selection
Medical‑grade polymers must meet biocompatibility standards (e.g. USP Class VI, ISO 10993), resist sterilisation, and maintain dimensional stability.
- Polycarbonate (PC): offers optical clarity and impact resistance—ideal for optical windows or reagent chambers.
- Polypropylene (PP): cost‑effective, chemical‑resistant and easily sterilised—commonly used for disposable fluidic paths.
- PEEK: used for components requiring mechanical strength and chemical resistance [Silkbridge Ltd, 2024].
Silicone, especially liquid silicone rubber (LSR), is frequently used in reagent seals or valves. Combined LSR and engineered plastics allow two‑shot moulding or over‑moulding for single, bonded components [NS Medical Devices, 2023].

2. Tooling Design and Manufacture
2.1 Tool Material & Manufacture
Steel (hardened tool steel) is standard for high‑volume, high‑precision applications, with aluminium acceptable for low‑volume or prototype tools. Standard CNC milling and Electrical Discharge Machining (EDM) are employed to achieve micron‑level detail [Wikipedia, 2023; Medical Moulds, 2023].
2.2 Number of Cavities & Production Volume
Tooling must reflect anticipated production volume. Single‑cavity tools are used for low volumes (≈ hundreds–thousands per year), while multi‑cavity or family tools improve per‑part economics for higher volumes [Creanova, 2021].
2.3 Conformal Cooling Channels
Incorporating conformal cooling channels in the mould ensures uniform cooling, reduces warping, and drastically shortens cycle time—critical where tight tolerances or microfluidic channels are concerned [Medical Moulds, 2023].

3. Moulding Process and Precision Control
3.1 Micro‑injection Moulding for Fine Features
For devices with microscale channels or volumes (e.g. microfluidic chips), micro‑injection moulding machines (shot weights 0.1–1 g) deliver tolerances ≤10–100 µm [Wikipedia, 2023].
3.2 Low‑shear, Clean Processing
Moulding parameters must minimise shear stress, residue, and fluorescence—especially in optical paths or surfaces contacted by reagents. Degassed polymer and gentle screw/barrel processing are essential [Freudenberg Medical, 2024; Medical Moulds, 2023].
3.3 Thin‑wall Moulding
Thin‑wall injection moulding techniques allow fast cycles, reduced material waste, and lightweight components—useful for disposable assay cassettes or syringe-like elements [Wikipedia, 2023].
4. Cleanroom Injection Moulding & Regulatory Compliance
PoC devices must be produced in facilities controlled to ISO Class 7 or 8 (ISO 14644‑1), using HEPA‑filtered air, gowning, and environmental monitoring to minimise particulate and microbial contamination [Medical Moulds, 2023].
Manufacturers must operate under a QMS certified to ISO 13485:2016, maintain full traceability (batch records, MES integration), and meet IVDR (EU), MDR, or FDA 21 CFR 820 frameworks [Medical Moulds, 2023].

5. Design for Manufacturability (DFM) and Assembly Integration
Tool design must accommodate:
- Gate and parting line placement to minimise flash and voids, optimise flow, and reduce trapped air.
- Draft angles to facilitate ejection without stress.
- Surface finish specs for fluidic pathways or optical clarity [Freudenberg Medical, 2024; Medical Moulds, 2023].
Moulded components should ease downstream assembly—e.g. pockets for membranes, snap‑fit areas for sensors, or locations for reagent freeze‑dry cake integration [ENL Ltd, 2023].
6. Validation, Quality Assurance and Scale‑Up
A robust validation protocol (including IQ/OQ/PQ) ensures process repeatability and reproducibility. Key process controls include in‑line dimension checks, leachables analysis, sterilisation qualification, and biocompatibility (ISO 10993) testing [Silkbridge Ltd, 2024].
For micro‑fluidic devices, machine‑vision or even ML‐based automated QC can be deployed to detect defects, pores, or geometric anomalies at scale [ArXiv, 2024].
7. Cost Efficiency and Strategic Tooling Choices
- Agile or rapid tooling (additively manufactured inserts, aluminium moulds) can shorten lead‑time and support prototyping, though with lower lifespan [Wikipedia, 2023].
- Multi‑cavity or family tools reduce per‑part cost when volume justifies tooling cost.
- Strategic tool design (e.g. modular inserts, interchangeable cores) enables iterative design without full re‑tooling [Creanova, 2021].
Production efficiency also benefits from runner‑less mould designs and material recycling, central to sustainability and cost control [Medical Moulds, 2023].
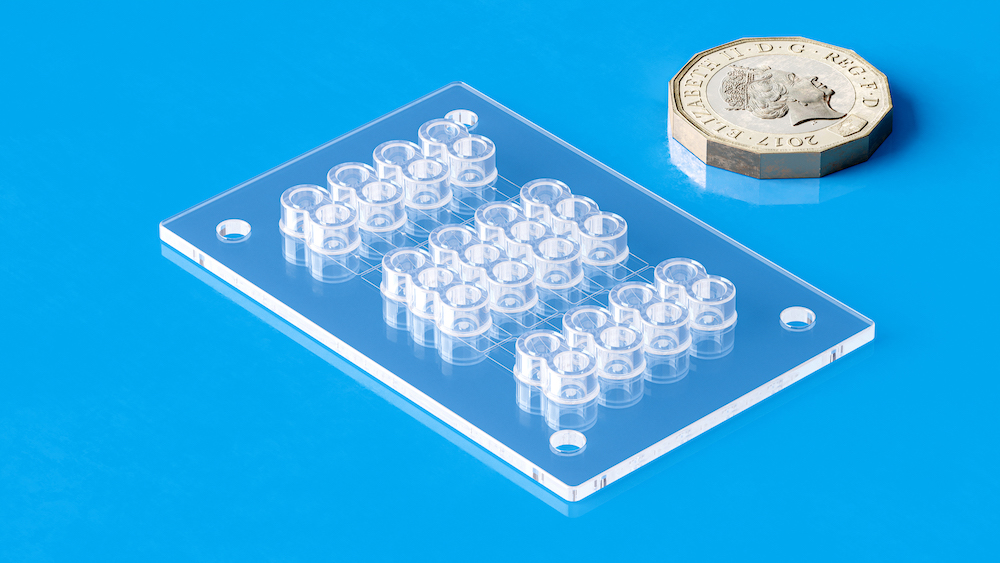
Tooling and moulding for PoC diagnostic devices require a multidisciplinary fusion of materials science, precision tooling, process engineering, regulatory knowledge, and cleanroom practice. From micro-injection moulding and conformal cooling to medical-grade polymers and ISO-compliant cleanroom facilities, every element must work in harmony to ensure diagnostic reliability and manufacturability at scale.
Micro Systems, a UK-based specialist in high-precision tooling and micro-injection moulding, provides a full-service solution for PoC diagnostic device manufacturers. With in-house capabilities covering tool design, micro-tool manufacturing, cleanroom moulding (ISO Class 7), and validated assembly processes, Micro Systems supports clients from concept development through to scalable production. Our expertise in microfluidic devices, thin-wall parts, and highly regulated markets ensures compliance and performance across the full product lifecycle.
Resources
- Silkbridge Ltd. (2024). Plastic Injection Moulding for the Medical Device Industry.
- NS Medical Devices (2023). Injection Moulding: Medical Applications.
- Medical Moulds (2023). Innovations in Micro-Injection Moulding.
- Medical Moulds (2023). Cleanroom Moulding and Regulatory Compliance.
- Freudenberg Medical (2024). Top 10 Considerations When Moulding Diagnostic Cartridges.
- Creanova (2021). How to Choose the Right Injection Mould Tooling.
- ENL Ltd (2023). Injection Moulding in Medical Device Manufacturing.
- Wikipedia (2023). Injection Moulding Technologies.
- ArXiv (2024). Machine Learning for Microfluidic Defect Detection.





