The True Value of Medical Injection Mould Tooling: Cost vs. Long-Term Benefit
In the world of medical injection moulding, the cost and quality of tooling can make or break a product’s success. Whether you’re manufacturing diagnostic devices, drug delivery systems, ophthalmic components, or surgical equipment, your choice of injection moulding tooling plays a pivotal role in ensuring precision, compliance, and scalability.
Although medical injection mould tooling can be expensive, the long-term advantages it brings to regulated, high-precision industries—like medical devices and pharmaceuticals—far outweigh the initial investment.
What Is Medical Injection Mould Tooling?
Injection moulding tooling refers to the custom-made steel or aluminium moulds used to produce high-precision plastic parts under pressure. In the medical industry, these tools must be engineered to exacting standards, capable of producing components that meet stringent requirements for functionality, sterility, and biocompatibility.
Unlike tools used in less regulated sectors, medical moulds are typically validated under ISO 13485 and must support comprehensive documentation, traceability, and repeatability.
Why High-Quality Medical Tooling Matters
1. Consistency and Precision
In medical injection moulding, even the smallest variance in a component can result in a failed device or regulatory issue. High-quality tooling ensures uniformity, tight tolerances, and reduced part variation—especially important for microfluidic devices, ophthalmic lenses, and drug delivery systems.
2. Regulatory Compliance
Under standards like ISO 13485, every element of the production process—including the tool itself—must be validated and documented. Investing in quality tooling helps simplify IQ, OQ, and PQ validation processes and reduces the risk of non-conformities during audits.
3. Tool Longevity and Efficiency
Premium medical moulds are designed to withstand high volumes over time with minimal maintenance. This durability reduces downtime and lowers the cost per part in long-term production runs.
4. Faster Time to Market
Well-designed, validated tools reduce the need for rework and design changes, helping companies bring their products to market faster—a key advantage in competitive and fast-moving fields like diagnostics and biotech.
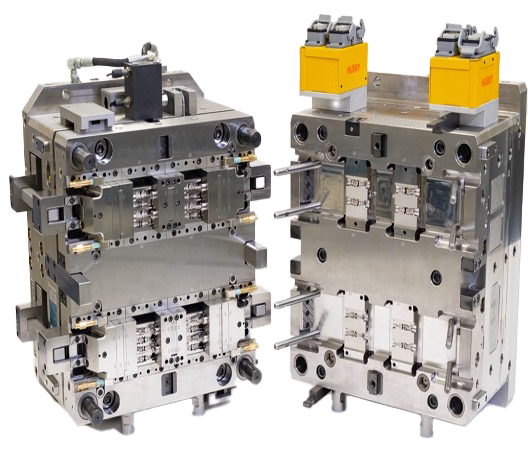
High-performance medical mould designed and manufactured by Micro Systems
How to Decide on Medical Injection Mould Tooling
Choosing the right tooling depends on a number of project-specific factors. Here’s how OEMs and product developers can make informed decisions:
Volume Requirements: Low-volume or development-stage projects may use prototype or bridge tooling. For full-scale commercialisation, investing in robust, multi-cavity production tooling is essential.
Component Complexity: Highly detailed or micro-scale parts require ultra-precision tooling, possibly with multi-component or insert moulding capabilities.
Validation Needs: Determine whether the tool must be validated for regulated use. If so, work with ISO 13485-certified partners to ensure smooth documentation and compliance.
Material Selection: Tools must be built to accommodate medical-grade polymers and withstand sterilisation methods such as autoclaving or gamma irradiation.
Lifecycle Goals: Consider whether the tool is needed for short-term use, or must support millions of cycles over several years. This will impact material choice, surface finishes, and overall investment.
Yes, Medical Injection Mould Tooling Can Be Expensive—But It’s Worth It!
There’s no denying the cost associated with high-quality injection moulding tooling—especially in the medical sector. However, cutting corners at the tooling stage often leads to greater long-term expenses in the form of failed validations, inconsistent parts, or product recalls.
Investing up front in a well-designed, fully validated medical mould pays off through improved product quality, faster regulatory approval, and reduced lifecycle costs. It also enhances brand reputation in a market where safety and reliability are non-negotiable.
Partnering for Success: UK-Based Tooling Expertise
Choosing the right tooling partner is as important as the tool itself. UK-based companies like Optimold, in collaboration with Micro Systems, offer a complete, ISO 13485 and ISO 9001-certified solution—from mould design and manufacture to full production and cleanroom moulding.
With world-class facilities in both the UK and Singapore, and expertise in micro and multi-cavity tooling, their integrated services help streamline the medical injection moulding process while ensuring global regulatory compliance.
 Micro Systems has world-class facilities for ultra-precision mould tooling
Micro Systems has world-class facilities for ultra-precision mould tooling
Medical injection mould tooling is a critical foundation for high-quality manufacturing. While the cost may seem high, the strategic value it offers in terms of precision, compliance, and efficiency makes it one of the most important investments in the product development lifecycle.
By working with experienced, ISO 13485-certified partners and making informed tooling decisions, companies can accelerate time to market, reduce risk, and deliver products that meet the highest standards of safety and performance.
Contact us today!





