Technical Advantages of UK-Based Manufacturing & Engineering in Medical Injection Moulding
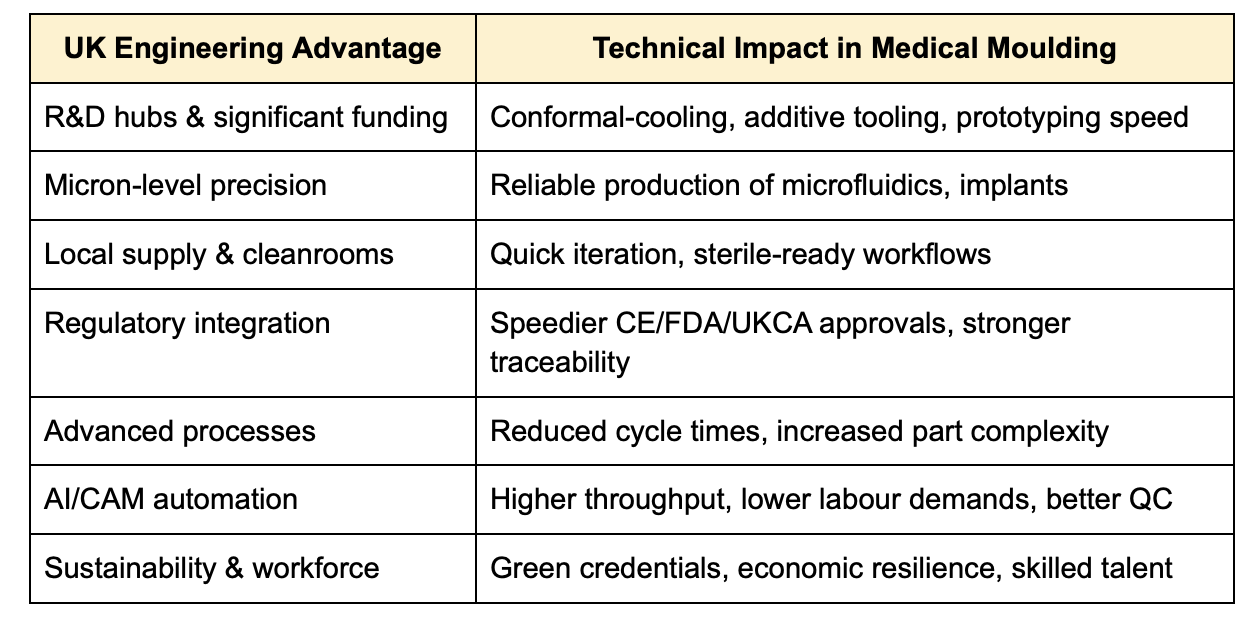
1. World-Class R&D & Innovation Infrastructure
- High Value Manufacturing Catapult network accelerates rapid prototyping and process development via engineering hubs across the UK (source).
- UK government support is strong: £2.8 bn is earmarked for advanced manufacturing R&D over five years, with life sciences receiving £600 m as part of the 10‑year industrial plan (source).
2. Precision Engineering Culture
- UK moulders achieve micron-level tolerances and intricate geometries essential for microfluidics, diagnostics, and implants (source).
- Clean, temperature-controlled production spaces (ISO Class 7) enable both tooling and injection in sterile environments (source).

World-class mould manufacture facility at Micro Systems
3. Agile Domestic Supply Chain
- Local tooling and production slashes lead times, supports iterative prototyping, and enables on-site clean-room manufacturing — critical for rapid clinical deployment (source).
- Domestic manufacturing minimises supply-chain disruption, reduces carbon footprint, and lowers transportation risks — especially vital during health crises .
4. Compliance & Regulatory Strength
- Most UK medical moulders hold ISO 13485/ISO 9001, ensuring controlled documentation, full traceability, and smooth CE/FDA/UKCA certification (source).
- Simplified regulatory pathways post-Brexit and proximity to MHRA, NICE, and NHS clinical networks aid faster market entry (source).
5. Advanced Tooling Techniques
- Techniques like conformal cooling channels, often CNC- or 3D-printed, dramatically reduce cycle times, minimise warping, and improve dimensional accuracy.
- Over‑moulding & insert‑moulding integrate multiple materials (metals, optics, seals) in single components, reducing assembly steps and increasing reliability .
6. AI & Automation Integration
- UK tech such as CloudNC’s AI‑powered CAM Assist cuts CNC programming time by ~80% and increases throughput tenfold, addressing critical labour constraints (source).
- Emerging Smart QC systems using machine-learning deliver near-100% defect detection accuracy in the injection moulding process .
7. Economic, Sustainability & Skilled Labour Benefits
- The UK medical device market is the 6th largest globally and 3rd in Europe (~$33bn), with ~3,500 companies and 86% NHS procurements (source).
- Government funding includes £520 m for life sciences manufacturing by 2030, and initiatives like the Life Sciences Innovative Manufacturing Fund support domestic capacity (source).
- Close supply chains reduce carbon emissions, and recyclable process optimisation supports green targets .
UK-based manufacturing and engineering bring unmatched technical precision, regulatory coherence, innovation-enabled tooling, and operational agility to the medical injection moulding sector. Backed by government investment and cutting-edge digital tools, the UK offers a resilient, high-quality, and sustainable manufacturing ecosystem tailored for critical healthcare applications.

Micro Systems (UK) is the premier partner for medical mould and injection moulding, offering unmatched precision (±0.001 mm) and in-house capabilities from ultra-precision toolmaking to cleanroom injection moulding (ISO Class 7). Our fully integrated UK facility supports rapid prototyping, micro-moulding of complex parts (down to milligrams), and production with medical-grade polymers like PEEK and COC. With ISO 13485 certification, regulatory expertise (CE/FDA/UKCA), and full traceability, Micro Systems delivers end-to-end solutions for critical medical devices—fast, reliably, and locally.
Contact us today!





