Mould Tooling for Class I, II, and III Medical Devices
In the medical device industry, injection moulding remains a critical process for producing components with the dimensional accuracy, repeatability, and material integrity required under stringent regulatory conditions. The tooling behind this process—specifically the mould design and build—is a defining factor in the successful, compliant, and cost-effective manufacture of medical devices across all FDA classifications.
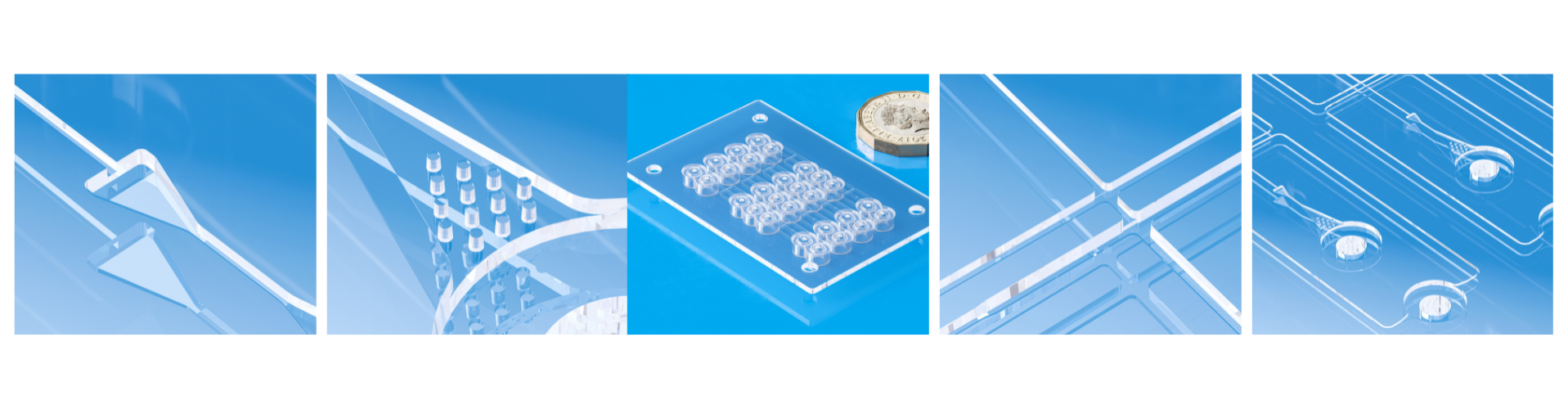
Medical devices like microfluidic chips require meeting strict global standards (photo: Micro Systems)
Overview of Medical Device Classifications
Medical devices are categorized by the U.S. FDA (and analogous global regulatory bodies such as the EU MDR and Health Canada) into three classes:
- Class I – Low-risk devices (e.g., disposable surgical tools, diagnostic swabs) with minimal regulatory controls.
- Class II – Medium-risk devices (e.g., syringes, cannulas, tubing sets) that require greater performance validation and design controls.
- Class III – High-risk or implantable devices (e.g., drug-eluting stents, pacemaker housings) that demand full premarket approval and exhaustive testing.
Each class dictates the degree of scrutiny placed on tooling precision, material compatibility, cleanroom requirements, and traceability.
Tooling for Class I Devices
High-Volume, Cost-Efficient, Standard Tolerances
While Class I devices are subject to fewer regulatory burdens, tooling must still meet basic Good Manufacturing Practices (GMP) under 21 CFR Part 820. Mould tools for these devices are typically designed for:
- High cavity counts (up to 96+)
- General tolerance control in the range of ±0.05 mm, depending on feature size
- Medium-hard tool steels such as P20 or H13 for cost-effective longevity
- Cycle time optimisation through advanced cooling channel design and ejection mechanics
- Minimal surface finish requirements (e.g., SPI B2 or B3) unless part interaction or appearance warrants otherwise
Design-for-manufacturability (DFM) is critical at this stage, as part complexity must be balanced with the need for efficient cycle times and ease of tool maintenance.
Tooling for Class II Devices
Tight Tolerances, Material Control, and Process Validation
Class II devices introduce additional technical and regulatory complexity. These tools must be capable of maintaining tight dimensional tolerances in the range of ±0.01–0.025 mm, particularly for mating components or drug delivery interfaces.
Tooling requirements include:
- Steel selection: High-hardness materials (e.g., S136, H13, or stainless steels) with corrosion resistance, particularly for processing medical-grade resins such as PC, PEBA, or medical-grade TPEs.
- Surface finishes: Polished or textured surfaces (e.g., SPI A2/A3 or custom EDM textures) to control release, reduce biofilm accumulation, or improve fluid dynamics.
- Ventilation and gating strategies: Precision venting and hot runner systems (valve gates preferred) to minimise flash, reduce shear, and maintain material integrity.
- Tooling documentation: Full design history file (DHF) support, including tooling IQ (Installation Qualification), OQ (Operational Qualification), and PQ (Performance Qualification) protocols.
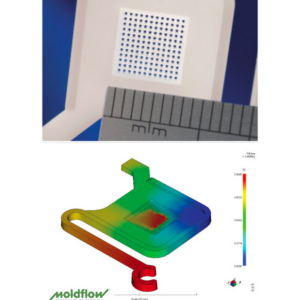
Micro Systems integrate Moldflow simulations to predict shear, warpage, and fill patterns—critical for devices that will undergo downstream bonding, sealing, or sterilisation.
Tooling for Class III Devices
Micron-Level Precision, Validation-Driven, Biocompatible Tooling Solutions
Class III devices, by nature, require the highest degree of precision, documentation, and risk mitigation. These devices often interface directly with critical biological systems or are permanently implanted, necessitating tooling that adheres to strict dimensional tolerances—frequently in the ±0.005 mm range or tighter.
Key engineering requirements:
- High-performance tool steels such as stainless S136H or Bohler M310 for maximum wear resistance and dimensional stability
- Mirror polish or SPI A1 finishes for implant-grade surfaces, especially in flow channels or optical features
- Tightly controlled shut-offs, often CNC ground or lapped to sub-10 micron tolerances
- Fully validated processes: Tooling must support full IQ/OQ/PQ validation along with documented Cpk studies and Gage R&R analysis to ensure part consistency
Additionally, tools must often be designed for integration into ISO Class 7 or 8 cleanroom environments, with features such as:
- Non-corrosive venting systems
- Self-contained ejector systems to minimise particulate generation
- Tooling that supports in-cavity sensors for real-time monitoring of pressure and temperature

Cleanroom micro moulding facilities at Micro Systems in the UK
Supporting Regulatory Compliance Through Tool Design
At Micro Systems, we not only design tooling for performance and longevity—we build it to support regulatory submissions and audits. Our in-house capabilities include:
- Turnkey Medical mould toolings and injection moulding solutions
- Full CAD/CAM design with documented DFM reviews
- Tool build documentation aligned with ISO 13485 and ISO 9001
- Mould qualification support (IQ/OQ/PQ) with traceable process parameters
- On-site injection moulding company Optimold for mould testing, validation labs and full-scale moulding services
For manufacturers serving the healthcare sector, understanding the specific tooling requirements for Class I, II, and III medical devices is essential. At Micro Systems, we specialise in designing and building high-performance mould tools tailored to meet the unique demands of each device classification, ensuring regulatory compliance and manufacturing excellence from prototype to full-scale production. From high-volume disposable components to ultra-precise implantable systems, our tools are engineered for compliance, performance, and total confidence—part after part, cycle after cycle.
Contact us today!





