Material performance requirements for self-injection pen device reliability
Reliable material selection and verification are central to the safety, functionality, and regulatory acceptance of self-injection pen devices. This article summarises the material performance requirements manufacturers should consider to ensure consistent dose accuracy, user safety, and long shelf life, mapping engineering needs to relevant standards and regulatory guidance. Key themes include mechanical performance, biocompatibility, chemical compatibility (extractables & leachables), sterilisation resilience, and real-world ageing (ISO 11608 [1]; FDA [2]; EMA/ICH [3]).
1. Regulatory and standards context
The ISO 11608 series defines functional and test requirements for needle-based injection systems (pen injectors), including mechanical and electronic performance tests. It is the primary consensus standard for design verification and type testing of pen injectors [1].
The FDA’s technical guidance on pen, jet, and related injectors provides considerations for device performance and recognises ISO standards while emphasising the need for device-specific data in submissions [2]. Combination-product guidance adds expectations for bridging studies, manufacturing controls, and material compatibility.
Biocompatibility and chemical safety are governed by ISO 10993 for biological evaluation and by regulatory frameworks such as FDA and ICH/EMA guidance for extractables and leachables [3][4].

2. Core material performance requirements
2.1 Mechanical and dimensional stability
Polymers and elastomers used for gearing, dose dials, plungers, and cartridges must maintain dimensions under stress and across the device’s claimed lifetime to preserve dose accuracy (ISO 11608-3 [1]).
Dimensional tolerance & creep resistance: Materials such as POM or PBT are preferred for consistent actuation and torque control.
Wear & friction behaviour: Sliding interfaces require stable friction; uncontrolled changes can affect dose delivery or user actuation force [5].
2.2 Environmental and sterilisation resilience
Materials must withstand the sterilisation process (gamma, e-beam, ethylene oxide, or aseptic manufacture) without losing mechanical integrity or producing degradation by-products [2].
Temperature and humidity cycling tests (accelerated and real-time) are recommended to verify retained function post-sterilisation [1].
2.3 Chemical compatibility and drug contact
Extractables and leachables (E&L) testing is essential for any drug-contacting material (cartridges, plungers, seals). The studies should follow risk-based E&L methodologies (FDA [4]; ICH Q3E [3]).
Extractables studies use solvent extraction to simulate worst-case scenarios, while leachables studies monitor migration into the actual drug product under storage conditions [4].
2.4 Biocompatibility
All materials contacting the patient or the drug must be assessed for biological safety per ISO 10993-1 [6]. The evaluation scope (cytotoxicity, sensitisation, systemic toxicity) depends on the type and duration of contact. Supporting literature and historical data may reduce testing where justified [6].
2.5 Long-term reliability and shelf life
Lifecycle and accelerated ageing tests must demonstrate reliable operation throughout the claimed use period. Functional tests for dose accuracy, actuation force, and mechanism durability are defined in ISO 11608 [1].
3. Typical material families and performance trade-offs
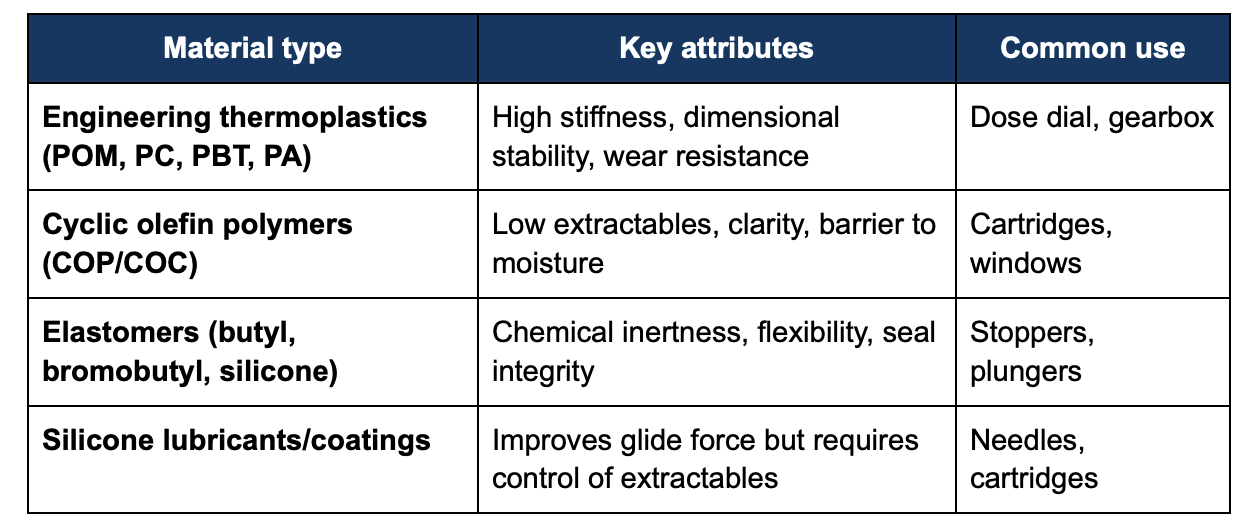
Silicone materials are particularly sensitive, as they can generate leachables or particulates if not properly controlled [5][7].
4. Verification and test strategy
A risk-based verification plan should include the following:
4.1 Material characterisation
Perform material identification (FTIR, DSC, TGA) and confirm batch consistency via supplier certificates and audits [2].
4.2 Functional performance tests
Test per ISO 11608-1/-3 for dose accuracy, actuation force, and mechanical lifecycle [1]. Deviations must be scientifically justified.
4.3 Environmental and accelerated ageing
Assess the impact of humidity, temperature, and UV exposure on mechanical performance. Results support shelf-life claims [1].
4.4 Extractables & leachables programme
Follow FDA [4] and ICH Q3E [3] methodologies:
Extractables: Use exaggerated conditions to profile chemical species.
Leachables: Evaluate drug contact over storage; align with toxicological thresholds.
4.5 Biocompatibility testing
Determine required tests per ISO 10993-1 [6], supported by justification from material use history and contact type.

5. Risk management and documentation
Apply ISO 14971 risk management principles to link material hazards (mechanical failure, toxicity, contamination) with mitigations and verification evidence. Maintain full traceability between specifications, risk files, and verification reports [8].
6. Practical development checklist
- Identify all drug- and patient-contacting materials and obtain full supplier disclosure.
- Conduct compatibility studies between materials and formulation under accelerated conditions.
- Execute E&L testing following FDA [4] and ICH Q3E [3] guidance.
- Complete biocompatibility evaluation per ISO 10993-1 [6].
- Perform ISO 11608-compliant mechanical and lifecycle testing [1].
- Demonstrate sterilisation compatibility [2].
- Establish change control and supplier quality agreements [8].

7. Common pitfalls
Ignoring silicone E&L impact: Silicone lubricants can release extractables; proper analytical control is required [5][7].
Assuming historical material safety: Evidence must support the equivalence of use and context [6].
Unlinked mechanical and chemical data: Combine mechanical ageing and E&L studies for robust justification [4].
8. Example material-to-test mapping
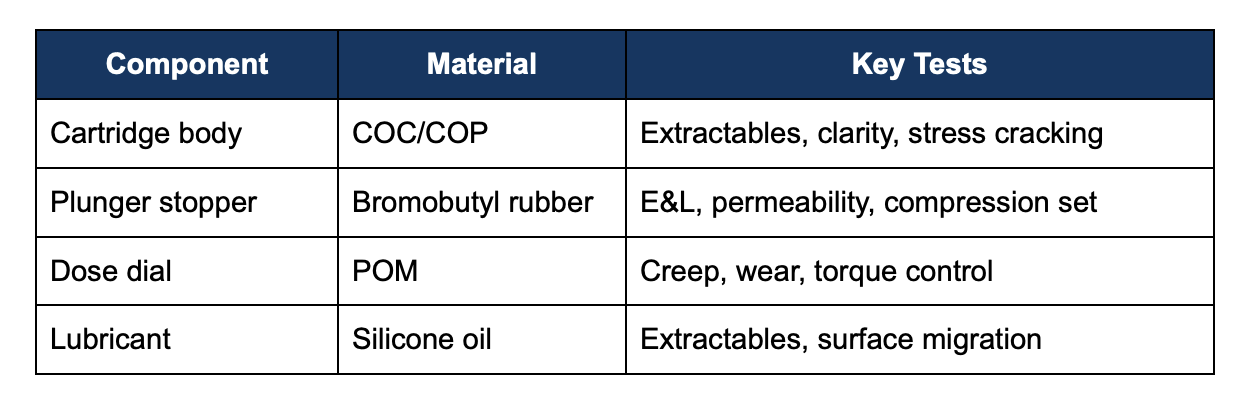
Conclusion
Material performance for self-injection pens integrates mechanical, chemical, and regulatory considerations. A rigorous programme combining ISO 11608 mechanical verification, ISO 10993 biological safety, and comprehensive E&L assessment ensures device reliability and global regulatory compliance. Early engagement between design, formulation, and regulatory teams is key to achieving robust, safe, and compliant drug-device products [1][2][3][4][6][8].
References
[1] ISO 11608 series — Needle-based Injection Systems for Medical Use (International Organization for Standardization).
[2] FDA — Technical Considerations for Pen, Jet, and Related Injectors Intended for Use with Drugs and Biological Products (U.S. Food and Drug Administration, 2023).
[3] ICH Q3E / EMA — Extractables and Leachables for Drug Products (International Council for Harmonisation / European Medicines Agency, 2024).
[4] FDA — Approaches to the Evaluation of Extractables and Leachables (2023).
[5] ISO 11608-3 — Test Methods for Functional Characteristics of Needle-Based Injection Systems (ISO, 2022).
[6] ISO 10993-1 — Biological Evaluation of Medical Devices — Part 1: Evaluation and Testing within a Risk Management Process (ISO, 2020).
[7] Zeng, Y. et al. “Impact of Silicone Lubrication on Drug Device Interaction in Pre-Filled Syringes,” Journal of Pharmaceutical Sciences, 2021.
[8] ISO 14971 — Application of Risk Management to Medical Devices (ISO, 2019).





