Micro Moulding and Miniaturisation in MedTech
The MedTech sector is undergoing a revolution with medical miniaturisation. Micro moulders are facing increased demand for small-scale components to enable innovative procedures beyond traditional injection moulding. Advancements in design, prototyping, and manufacturing continue to expand the possibilities, proving that “smaller is better” in this field.
Lab-on-a-chip with 300 μm micro channels and chambers with static micro mixing features, and 3 LUER cones (Photo: Micro Systems)
What is Miniaturisation?
Miniaturisation is the process of reducing the size of a tool or equipment, generally for mechanical, optical and electronic products and devices. Over the last 50 years, med-tech pioneers have been exploring methods to shrink components to smaller dimensions within the micro range, and moving into the nanometer range.
Miniaturisation brings a number of benefits for patients, clinicians and suppliers.
Size: Smaller devices mean less space required for storage and transportation, hence related tasks like handling, management are also less time-consuming.
Cost: The smaller size of miniaturised medical devices helps reduce costs for both healthcare providers and patients, as less intrusive procedures lead to shorter hospital stays and faster recovery. Additionally, the compact design reduces storage and transportation needs, resulting in further savings, particularly in unit transport costs.
Convenience: Smaller devices enable less invasive procedures, reducing surgical trauma, hospital stays, and follow-up visits. Miniaturisation allows at-home diagnostics, such as with swallowable cameras, saving time for both patients and clinicians. More features in compact tools also support better diagnostics and improved patient care.

How to achieve Miniaturisation in MedTech?
With technology advancing rapidly, designers and manufacturers are making miniaturisation possible in more devices within the Medtech industry.
Due to the specialised nature of medical device manufacturing, companies typically work closely with clients from the outset to prioritise human-centred design, functionality, and cost-efficiency. At Micro Systems, for example, dedicated teams collaborate with clients in an ISO-certified mould testing and development facility, integrating injection moulding machines, inspection systems, robotics, and assembly equipment to provide a complete turnkey solution. This close partnership enables manufacturers to achieve Design for Micro Moulding, optimising design, reducing costs, and shortening lead times through improvements in design, material selection, manufacturing processes, and testing.
Material selection is crucial in medical micro moulding, with common choices including thermoplastics like ABS, LCP, PC, PE, PEEK, and bioabsorbable materials. Each material behaves differently under the high pressures, velocities, and rapid cooling rates of micro moulding, affecting their mechanical and thermal properties. Defects such as voids or poor knit lines can also lead to component failure due to reduced polymer strength.
Key considerations in material choice include environmental conditions (e.g., temperature and pressure), design requirements (e.g., thin walls), end-use (e.g., implantable or bio-contacting), the impact of additives (e.g., glass or carbon), and cost (bioabsorbable materials tend to be more expensive). For example, PEEK offers excellent mechanical properties and biocompatibility but requires precise control over mould design and process, particularly for thin-walled structures, compared to materials like LCP or polypropylene.

High-tech equipment and strict standards are commonly required for micro moulding projects (Photo: Micro Systems)
Next, with more complicated added features, a higher complexity of the manufacturing process, tooling and equipment is required. As generally, micro moulding requires different aspects compared to conventional moulding, specific micro moulding suppliers need to heavily invest in ultimate precision mould manufacturing machines, inspection equipment, and other specific requirements for the medical industry like class 7 cleanroom. To regulate material flow into the mould cavity, micro moulding tools must have precise tolerances and shut-off surfaces. This requires highly skilled toolmakers with expertise in micro moulding. The design team at Micro Systems has successfully managed complex projects, including selecting specialist steels for optical surfaces, micro-machining, and optimising high-temperature heat transfer for specific micro features. To ensure client sustainability, Micro Systems’s moulds are built for durability, running between 3.5 to 5 seconds for up to 40 million cycles before major refurbishment is needed.
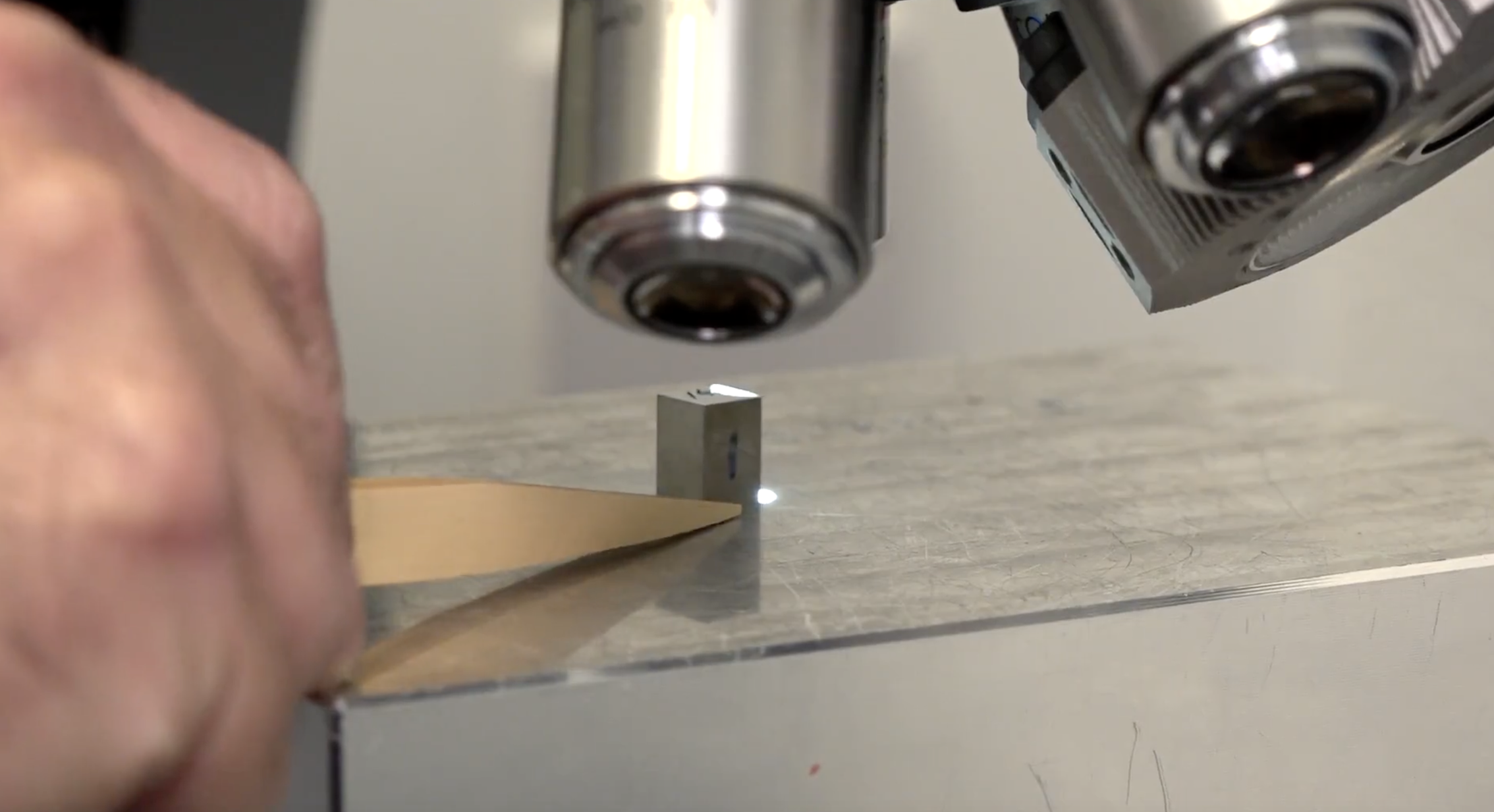
Finally, validation is crucial. The complexity of process control and the challenges of measuring, manipulating, and inspecting such small features make it difficult to validate micro moulded components. Precise inspection is essential before parts are delivered to customers, requiring highly accurate metrology. At Micro Systems, for example, we utilise CNC CMM systems with 1-micron capability, confocal non-contact measurement systems in the nanometre range for profile and surface finish measurements, and CT-Scan CAD-Compare technology to accurately and consistently measure micron-scale features and optical surfaces.
Once inspected, micro moulded components must be handled and packaged with great care, as their small size and potential need for specific environmental conditions—particularly in medical applications—mean traditional handling methods may not be suitable.
Given the complexity of micro moulds and the rapid evolution of moulding technology, it is often more advantageous for OEMs to outsource their micro moulding projects to specialists with unique technical expertise. In particular, when dealing with micro production, where tolerances and detail are critical, in-house tooling, moulding, assembly, and testing are essential. A turnkey approach with a single mould supplier enhances transparency, reduces risk, minimises time and financial waste, and results in optimised products.
References:
Fuges, C. (2021) Toolroom perspective on Micro Molding, MoldMaking Technology. Available at: https://www.moldmakingtechnology.com/articles/toolroom-perspective-on-micro-molding (Accessed: 17 May 2023).
Micromolding: Small Parts, big challenges (no date) Canadian Plastics. Available at: https://www.canplastics.com/features/micromolding-small-parts-big-challenges/ (Accessed: 17 May 2023).
13, M. (2016) The challenges of micromolding, plasticstoday.com. Available at: https://www.plasticstoday.com/challenges-micromolding (Accessed: 17 May 2023).
How precision tooling can make miniaturisation possible (2023) Medical Plastics News. Available at: https://www.medicalplasticsnews.com/medical-plastics-industry-insights/latest-medical-plastics-insights/how-precision-tooling-can-make-miniaturisation-possible/ (Accessed: 17 May 2023).
Micro Systems, based in Golbourne, Greater Manchester, UK, has been a leading supplier for micro moulding, mould design, mould manufacture and metrology, for major OEMs in the medical, pharmaceutical, ophthalmic, packaging markets and many more. Micro Systems has a range of the latest ultimate precision mould manufacturing machines and equipment, with state-of-the-art facilities in both the UK and Singapore, serving customers across continents with millions of fully tested and manufactured micro components and custom micro moulds. For more information, please Contact us or visit our website.





